Hf Vsepr Shape
By signing up you ll get thousands of step by step solutions to your homework questions.
Hf vsepr shape. The valence shell electron pair repulsion theory vsepr as it is traditionally called helps us to understand the 3d structure of molecules. Hcn molecular geometry is linear. A combination of vsepr and a bonding model such as lewis electron structures is necessary to understand the presence of multiple bonds.
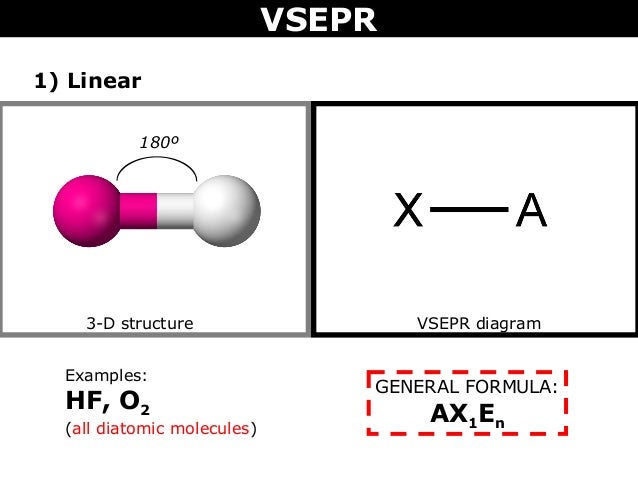
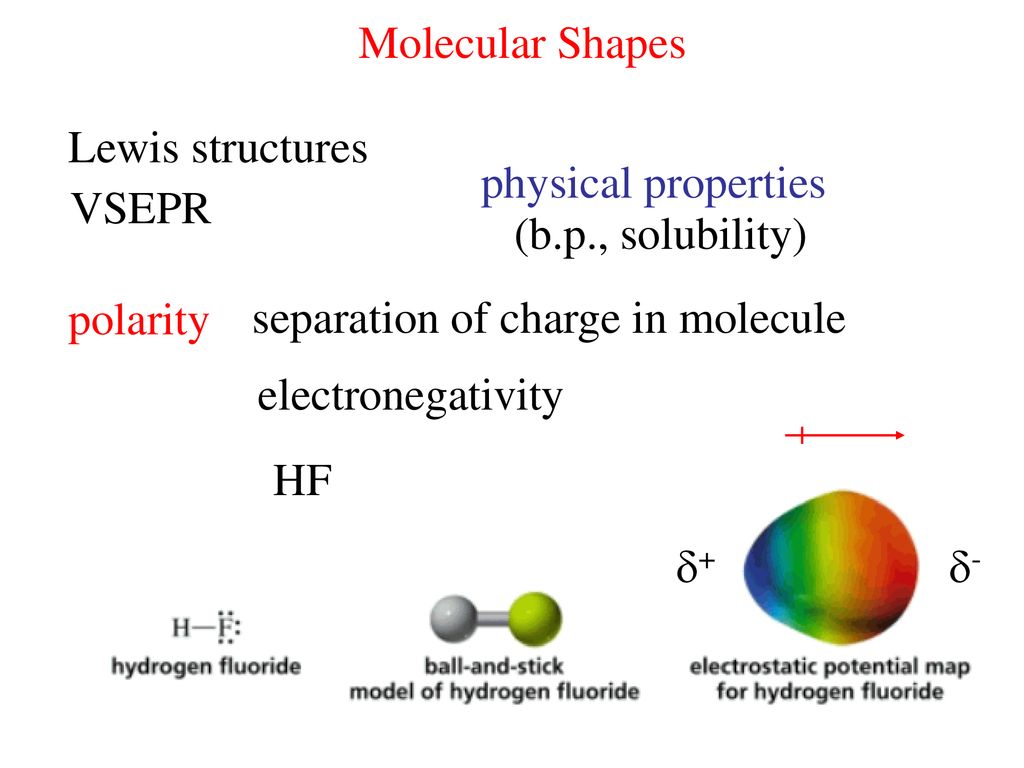
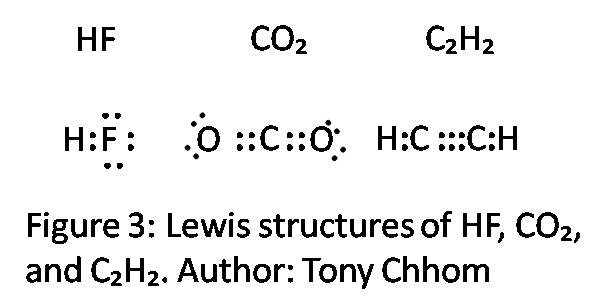
I m having trouble with this because how can there be a central atom with only 2 atoms. The vsepr model states that the electron regions around an atom spread out to make each region is as far from the others as possible. What is the molecule shape of hf and how many atoms are bonded to the central atom and how many are lone pairs.
For hf there is a larger dipole moment because there is a larger difference in electronegativity. The vsepr theory predicts that xef is linear. Although we will speak often of electron pairs in this discussion the same logic will hold true for single electrons in orbitals and for double bonds where one could think of the bond as consisting of two pairs of electrons.
Valence shell electron pair repulsion theory vsepr theory. Vsepr theory for hf please help. There is a small dipole moment.
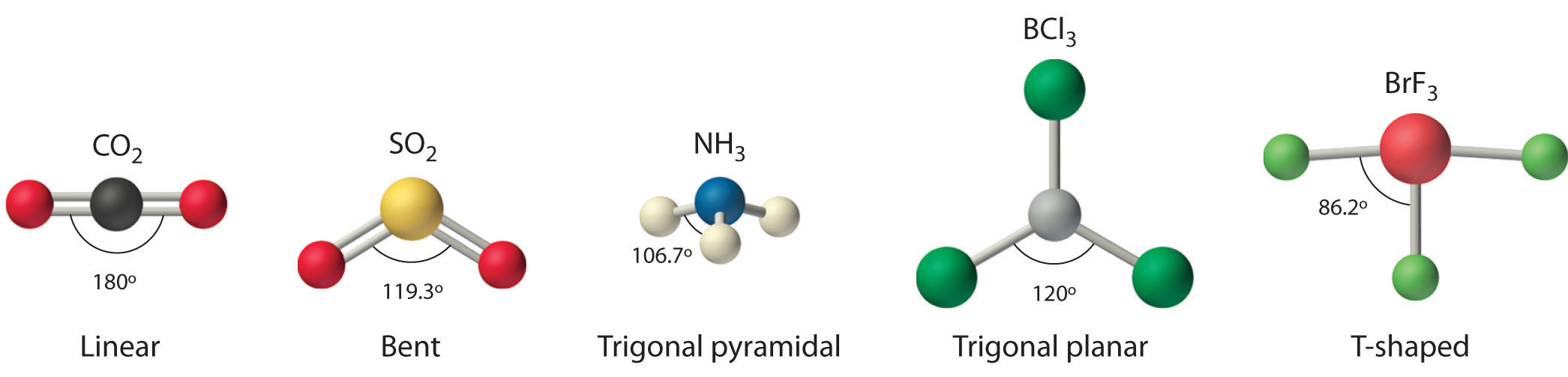
A x and notation theory can also be used to make sure about the right molecular geometry. According to vsepr theory which molecule has a bent shape co2 h2o cs2 hf. What is the electron geometry of f 2 hf.
When a molecule contains more than one bond. They are the three lone pairs and the two xe f bonds. Note that the vsepr geometry indicates the correct bond angles.
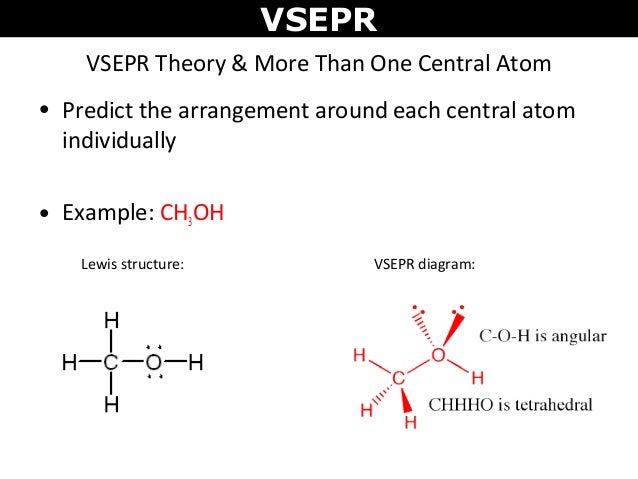
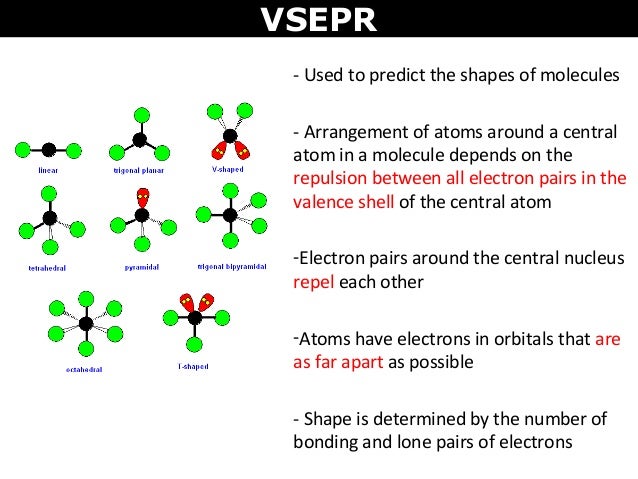
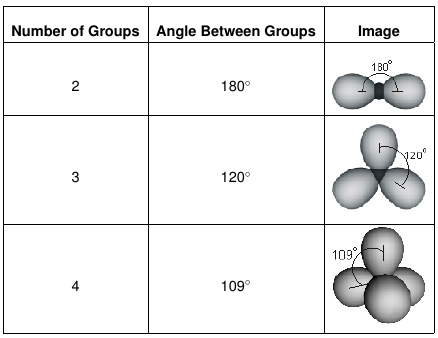
We can use the vsepr model to predict the geometry of most polyatomic molecules and ions by focusing on only the number of electron pairs around the central atom ignoring all other valence electrons present according to this model valence electrons in the lewis structure form groups which may consist of a single bond a double bond a triple bond a lone pair of electrons or even a single. This tells us that there are five electron regions steric number 5 about the central carbon atom. The vsepr model can be used to predict the shapes of many molecules and polyatomic ions but it gives no information about bond lengths and the presence of multiple bonds.