Lewis Structure Of Two Hf Molecules
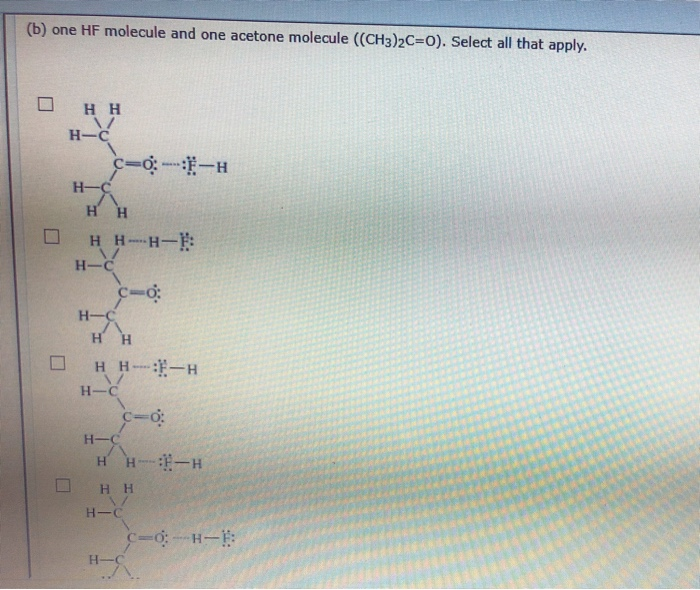
Write the lewis structures for the two molecules.
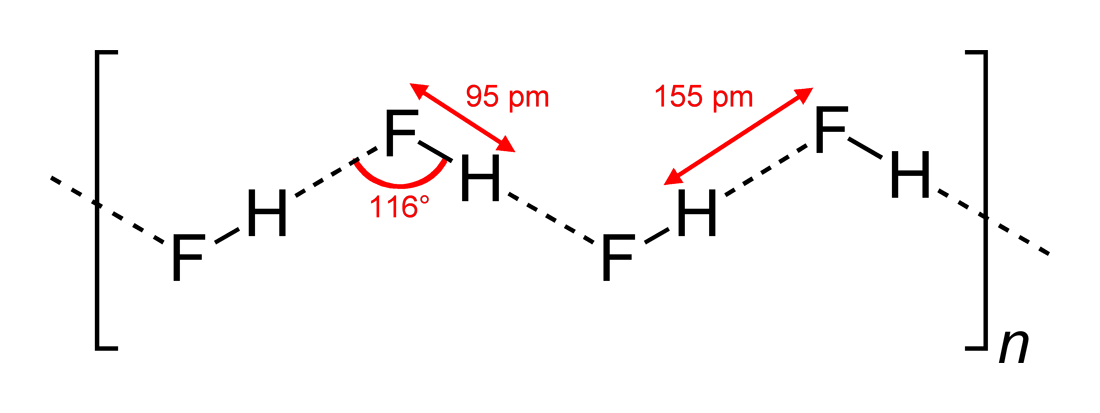
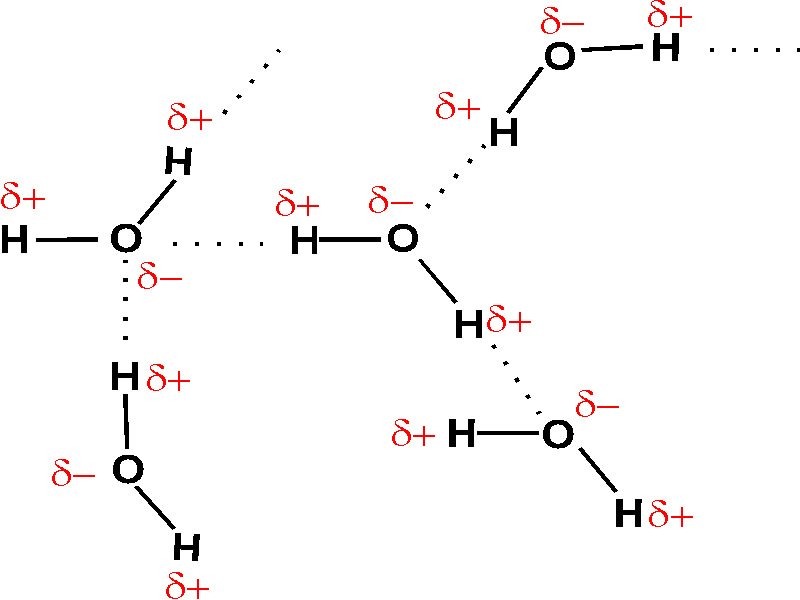
Lewis structure of two hf molecules. When two hydrogen fluoride molecules interact with each other then they form a zig zag structure involving interaction between positively charged hydrogen of one molecule with negatively charged fluoride of another molecule 5. A step by step explanation of how to write the hf lewis structure hydrofluoric acid. A compound with a molar mass of about 42 g mol contains 85 7 carbon and 14 3 hydrogen by mass.
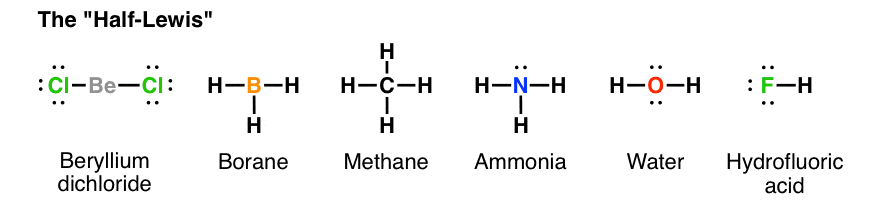
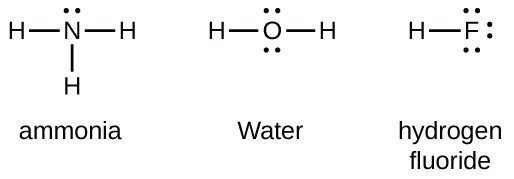
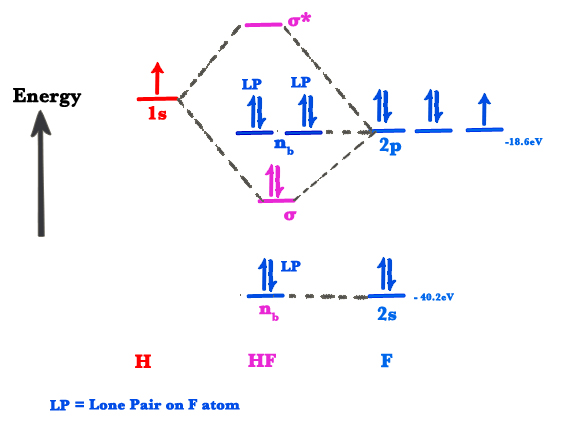
Hydrogen fluoride hf is a compound that is primarily polar. These hf molecules further make chains with each other through hydrogen bonding interactions. First we count the valence electrons for hf using the periodic table.
The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom. Two arrangements of atoms are possible for a compound with a molar mass of about 45 g mol that contains 52 2 c 13 1 h and 34 7 o by mass. This is due to the high electronegativity of the fluorine that pulls the shared electron pair between h and f more towards its side.
A lewis structure also helps to make a prediction about the geometry of a molecule. This leads to the development of a partial negative charge on the f atom and a partial positive charge on the h atom leading to the generation of a dipole and hence polarity. The tendency of main group atoms to form enough bonds to obtain eight valence electrons is known as the octet rule.
One single bond between atoms and three lone pairs of electrons per atom this allows each halogen atom to have a noble gas electron configuration. Hydrogen fluoride is a chemical compound with the chemical formula h f this colorless gas or liquid is the principal industrial source of fluorine often as an aqueous solution called hydrofluoric acid it is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers e g.